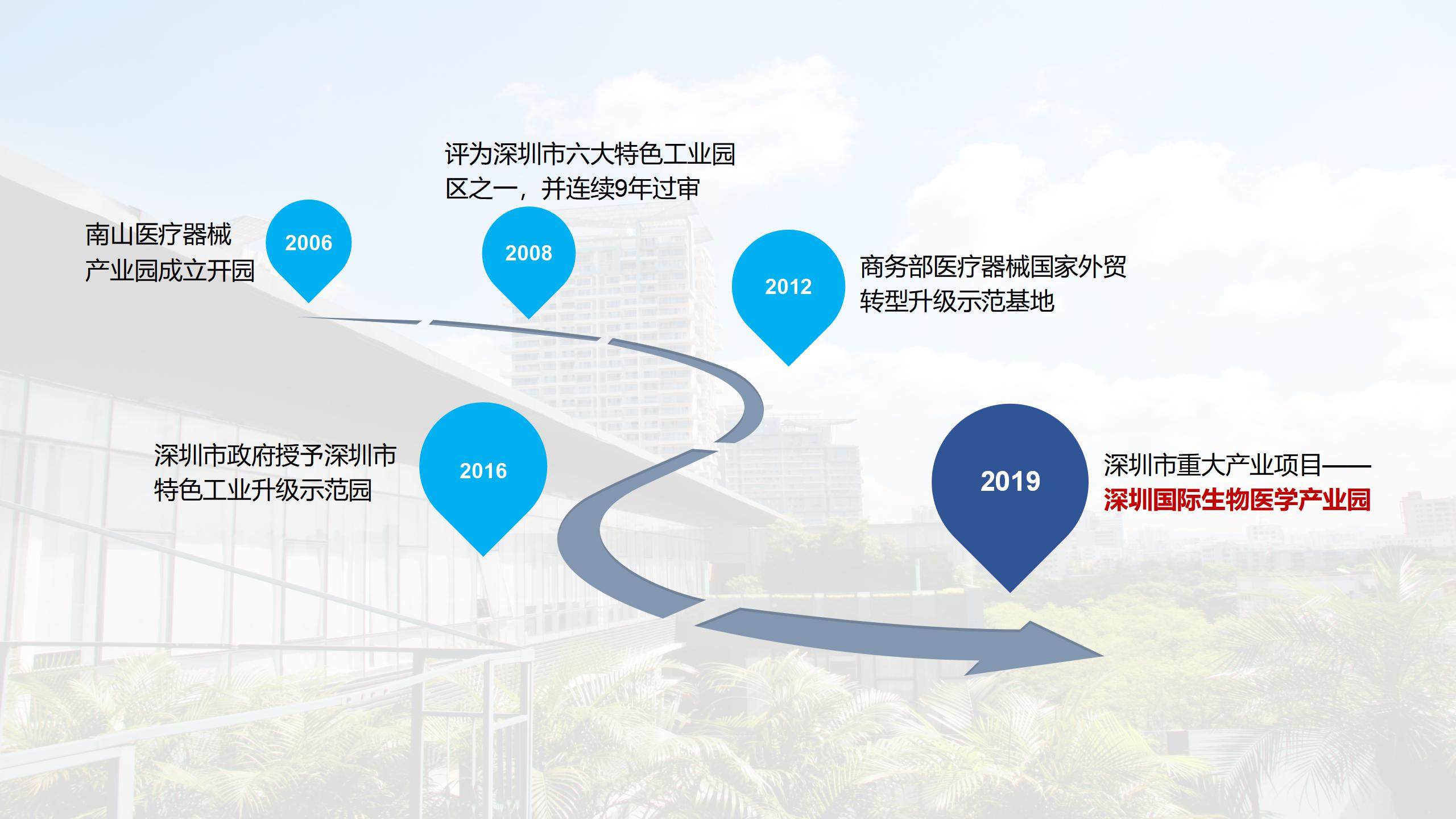
We will take full advantage of the medical device registration system in China, make full use of the R&D team's strength and its professional and efficient services, form an organic ecosystem by connecting various elements, improve the success rate of innovations, expand the number of innovation projects, and create a new professional park integrating “industry-university-research-investment-application sectors”. We will take advantage of the once-in-a-lifetime opportunity to turn Shenzhen into a pilot demonstration zone, as well as its geographic advantages in the free trade zone, to transform technological achievements and establish an incubation base for high-end medical devices, strive to become a highland of medical device registration certificates in the Greater Bay Area (Guangdong-Hong Kong-Macao ) within three years, and assist Shenzhen in taking the lead in the Greater Bay Area's new wave of innovative medical device development.