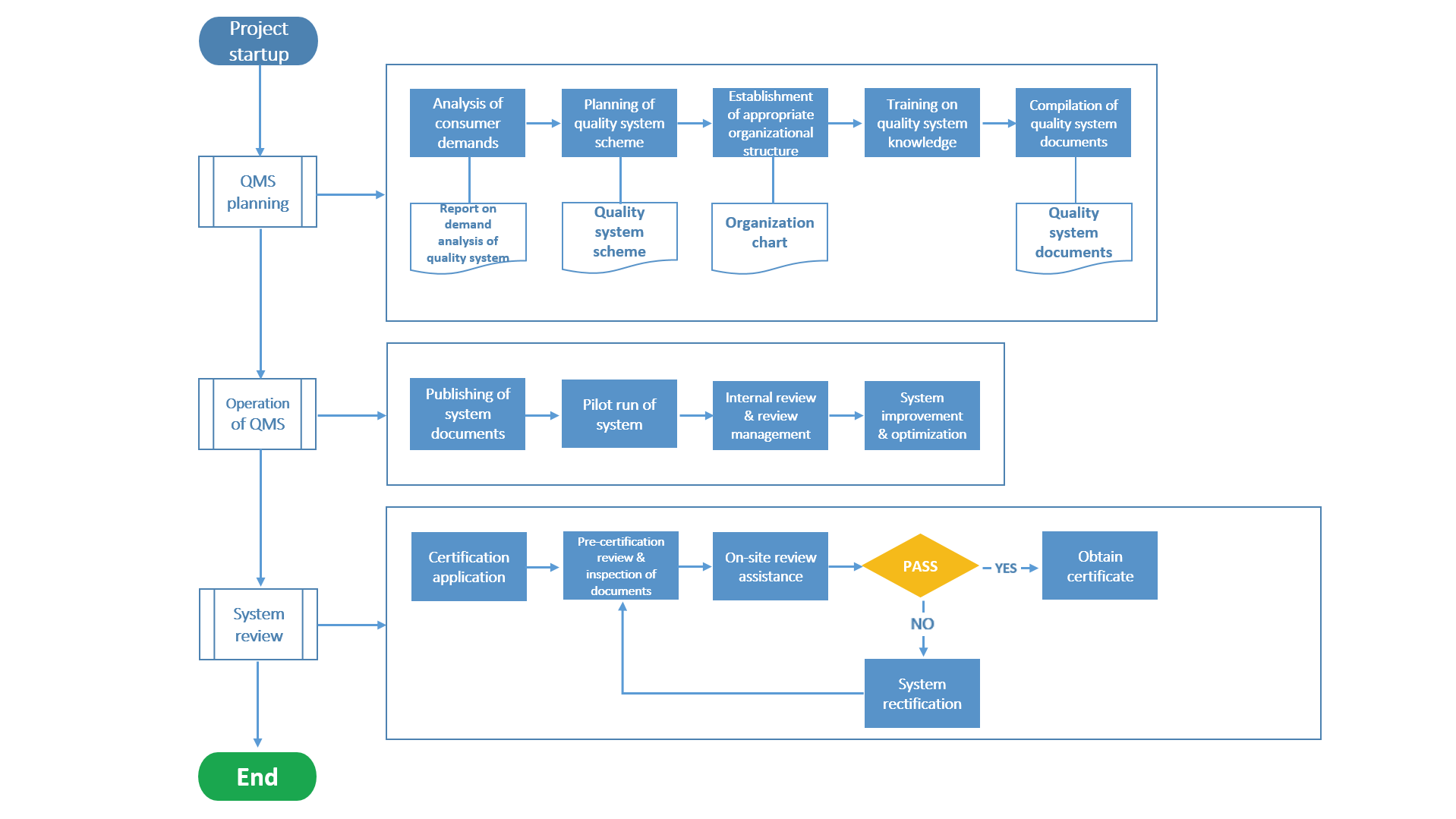
Medical Devices-Quality Management Systems-Requirements for Regulatory Purposes (ISO13485) is the most authoritative international quality system standard in the medical device industry, covering product R&D, production, quality inspection, sales and after-sales and other aspects, the establishment of which aims to ensure that mandatory requirements are met with medical device products before entering the market for circulation, and it is an important means to realize the control of medical devices in their full lifecycle and ensure their safety and effectiveness.
MGS Life Sci-Tech provides regulation consultation and system assistance services in terms of global multinational quality management systems (ISO13485, QSR820, etc.), production quality management standards for medical devices (asepsis, implantation, IVD, life support, rehabilitation of chronic diseases) and other aspects for medical device enterprises, assists them to streamline their processes of “system management - design and development - production site - quality improvement - sales and after-sales standards”, and builds a streamlined, efficient, legal and compliant system process foundation for the full lifecycle management of products.